Abstract
BACKGROUND. HCV-positive DLBCL has distinct clinical and pathologic characteristics compared to its negative counterpart: patients (pts) are usually older with more frequent splenic and extranodal involvement and elevated LDH. Differently from its clinical hallmarks, the molecular landscape of this pathological entity has been scarcely outlined.
METHODS. In this bicentric study, we investigated the clinical and molecular features and outcome of 54 pts with HCV-positive DLBCL. Targeted next generation sequencing (NGS) was performed on DNA extracted from formalin-fixed paraffin-embedded tissue biopsies. A core panel probes covering coding exons from 184 genes frequently mutated in mature B cell neoplasms was designed using IDT tool and libraries were prepared using Illumina DNA-prep-with enrichment. Sequencing was performed on Illumina HiSeq 2500. Cluster analysis was performed using LymphGen tool. We also applied fluorescence in situ hybridization (FISH) for MYC, BCL2 and BCL6.
RESULTS. Median age was 71 (33-84; IQR: 61.9-77). Stage was III/IV in 34 pts (63%). Extranodal sites were involved in 21 pts (38%), spleen in 20 pts (37%). LDH was higher than the upper limit in 40 pts (74%). R-IPI was good for 2 pts (4%), intermediate for 24 pts (44%), poor for 28 pts (52%). HPS score was intermediate or high in 33 of 44 assessed pts (75%). A histological low-grade component was identified in 15 pts (27%). Hans algorithm differentiated pts almost equally in GCB (26/50, 52%) and non-GCB (24/50, 48%) subtype. HCV-RNA was detectable in 52 pts (96%) and quantifiable in 43 pts (79%). Of 29 pts assessed, genotype was 1 in 9 (31%), 2 in 16 (55%), 3 in 4 pts (14%). Among 37 pts whose data were available, 11 pts (30%) received direct antiviral agents, 7 pts (19%) received interferon-containing regimen, 19 pts (51%) were not treated for HCV. Twenty-seven pts (50%) received rituximab-enriched protocols, 23 pts (43%) were treated with chemotherapy alone, 1 pt (2%) with surgery alone, 3 pts (5%) were lost to follow up. With a median follow up of 7.7 years (yrs) (IQR: 4.6-10.6), 5-yrs overall survival (OS) (95%CI) was 49.3% (34.1-62.8%) and 5-yrs progression free survival (PFS) (95%CI) was 39.5% (25.5-53.3%). Median OS and PFS were 4.9 and 3.1 yrs, respectively. FISH analysis showed lack of BCL2 (0/19) and MYC translocations (0/15). BCL6 fusions were found in 76% of pts (16/21).
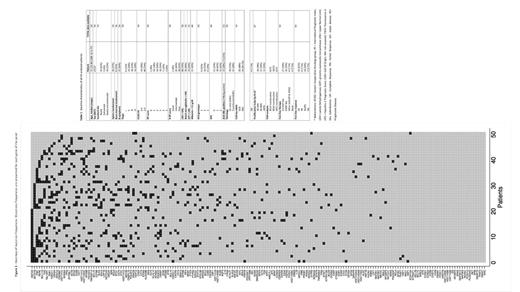
NGS showed mutations in 154 of the 184 analyzed genes. The informativity of the panel was 100% with all pts presenting at least one oncogenic variant. Gene mutation frequencies are presented in Fig. 1. The median mutation load (MML) was 13 mutated genes per case (2-32; IQR: 9-16). Most frequently mutated genes were the epigenetic regulators KMT2D, mutated in 23 pts (42.6%), and SETD1B, mutated in 17 pts (31.5%). FAS, PM1 and RERE were mutated in 15 pts each (27.8%). TBL1XR1, BCL11A and SGK1 were mutated in 14 (26%), 13 (24%) and 12 pts (22%), respectively. Considering genes in their specific pathway, 94% of pts harbored mutations in genes involved in epigenetic regulation (MML: 3; range 1-7; IQR: 1.25-4), 90% of pts in apoptosis-related genes (MML: 2; 1-7; IQR: 1-3) and 77% of pts in genes belonging to BCR/NFkB signaling pathway (MML: 2; 1-7; IQR: 1-3). Of note, 56% of pts carried mutations in genes related to immune regulation (MML: 1.7; 1-5; IQR: 1-2) and 25% of pts had mutations within the NOTCH pathway (MML: 1.2; range 1-2). Via the LymphGen 1.0 tool, we classified 26 pts (48%) into 4 genetic clusters: BN2 (11/26, 42%), ST2 (8/26, 31%), MCD (4/26, 15%), EZB (3/26, 12%). Twenty-eight pts (52%) were classified as "others". Among those belonging to BN2 cluster, 7 pts (64%) had a histologically confirmed transformed DLBCL. No significant differences in terms of OS and PFS were identified according to cluster subgroups.
CONCLUSIONS. The prevalence of the BN2 cluster and enrichment of mutations of genes involved in NOTCH pathway seem to indicate a preferential marginal-zone origin in HCV-positive DLBCL. In addition, our data confirm the absence of BCL2 translocation in this subset of DLBCL and show a high prevalence of mutated genes within the epigenetic and immune regulation pathways in HCV-positive DLBCL, pointing out their compelling role in the pathogenesis and suggesting potential implications for molecularly targeted therapies.
Passamonti: Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Arcaini: Celgene: Speakers Bureau; Gilead Sciences: Research Funding; Bayer, Celgene, Gilead Sciences, Roche, Sandoz, Janssen-Cilag, VERASTEM: Consultancy; Celgene, Roche, Janssen-Cilag, Gilead: Other: Travel expenses.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal